How Many Valence Electrons Does Astatine Have
2 Valence Electrons Radium atoms have 88 electrons and 88 protons with 2 valence electrons in the outer shell. So they do 8 the number of total.
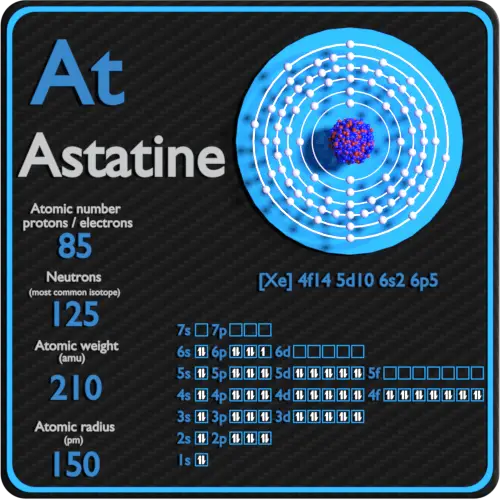
Astatine Protons Neutrons Electrons Electron Configuration
Nickel has eight electrons in the 3d orbital and two electrons in the 4s orbital which means nickel has 10 total valence electronsThe reason it has 10 is because nickel is a transition metal so the d and s electrons can participate in chemical bonding.

. Helium atom has 2 electrons in its valence shell but its valency is not 2. It is produced in a cyclotron by bombarding Bismuth-209 with alpha particles. Ok but how many valence electrons does an atom of Astatine have.
It has seven valence electrons. Is valency and valence electrons the same. All of its isotopes have a very short half-life which makes it difficult to mine ore extract.
1 day agoLets say we take Helium which has 2 electrons. Possible oxidation states are. Anonymous May 25 2016 The element Astatine has 85 protons as well as 85 electrons.
How many valence electrons does astatine have. How can you determine the number of valence electrons in an atom. For main group elements ie s-block and p-block.
Astatine Overview Astatine Valence Electrons 1357 Atomic Number 85. In the periodic table the elements are listed in order of increasing atomic number Z. In the case of Astatine the valence electrons is 1357.
How many valence electrons does group 15 have. Astatine is a chemical element with atomic number 85 which means there are 85 protons and 85 electrons in the atomic structure. 119 rows Valence electrons.
Astatine has a valency of 1 because it is in group 7 and is part of the halogens. Any element in the halogen group will have seven valence electrons. Sodium Na has 11 protons using its atomic number that is found on the.
The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Keeping this in view how many valence electrons are in an atom. Therefore the valence electrons of argon Ar are eight.
These elements include fluorine chlorine bromine iodine and astatine. Where is astatine found. Any element in the halogen group will have seven valence electrons.
The electron configuration of the gallium shows that the last shell orbit of gallium has a total of three electrons. Valency is the number of bonds an element or an atom can form. They are in 6s2 6p5 orbitals.
That is we can easily say that the valence electrons of gallium are three. Astatine has no stable isotope. Electron configuration of Astatine is Hg 6p5.
The number of electrons in each elements electron shells particularly the outermost valence shell is the primary factor in determining its chemical bonding behavior. The total number of electrons in a valence shell is called a valence electron. They say the group number is the number of valence but the valency cant be 7.
The main group number for an element can be found from its column on the periodic table. Radium is a silvery metal. The electron configuration of argon shows that the last shell of argon has eight 3s 2 3p 6 electrons.
Now lets check the facts about Astatine. The number of protons is equal to the atomic number the big number by the atom on a periodic table The number of electrons is equal to the number of protons The number of neutrons is equal to the atomic mass minus the atomic number Denise. The last shell after the electron configuration is called the valence shell.
For neutral atoms the number of valence electrons is equal to the atoms main group number. Answer 1 of 3. The 31st element in the periodic table is gallium.
An atom has 6 electrons in its outer shell. Since astatine is a halogen it follows the same pattern as the other halogens and has seven valence electrons. Group 15 has 5 valence electrons.
The chemical symbol for Astatine is At. A valence electron is an outer shell electron and may participate in the formation of a chemical bond. These elements include fluorine chlorine bromine iodine and astatine.
Click to see full answer. They have seven valence electrons so they are very eager to gain one Halogens are highly reactive nonmetallic elements in group 17 of the periodic table. For neutral atoms the number of valence electrons is equal to the atoms main group number.
That is the atom of the gallium element has a total of thirty-one electrons. For example carbon is in group 4 and has 4 valence electronsOxygen is in group 6 and. The nucleus is composed of protons and neutrons.

2022 Valence Electrons In Astatine At Facts Color Discovery

Astatine Facts Symbol Discovery Properties Uses

Astatine Atomic Structure Stock Image C018 3766 Science Photo Library
No comments for "How Many Valence Electrons Does Astatine Have"
Post a Comment